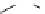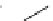Biomedical Engineering Reference
In-Depth Information
Me
TBSO
Me
OH
PMBO
N
*
O
2
N
*
H
*
Boc
O
Build
Me
O
6
8 stereoisomers
Me
*
28
2 stereoisomers
Couple
Appendage Diversity Sites
PMBO
Me
O
e
*
5
Me
Me
2
6
PMBO
N
*
Me
N
2
*
*
N
Boc
N
*
*
5
O
2
N
Boc
O
2
N
Me
O
O
6
O
Pair
O
O
*
*
Me
12
Me
12
29
30
16 stereoisomers
16 stereoisomers
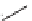
FIGURE 17.11
Preparation of macrocycles using the build/couple/pair strategy.
with 560 compounds, and titration at a four-point dose further narrowed the list
to 26 candidates. Of these compounds, 20 compounds were from the ring-closing
metathesis (RCM) macrocyclic library, and this scaffold was selected for further
studies. The primary hit compound was titrated in a 12-point assay to give a potency
(EC
50
) of 120 nM against Dd2 erythrocytic parasites. The RCM library is a series
of 14-membered macrocycles with four stereogenic centers. This chemotype has
no parallels in malaria therapy, and indeed, macrocyclic rings are often underrepre-
sented in traditional screening collections, probably because their syntheses are often
considered difficult to develop.
The RCM library was prepared using a B/C/P strategy and involved an initial
build phase of chiral building blocks using an asymmetric aldol reaction and chiral
reagents (Figure 17.11). The carboxylic acid (
28
) and amine (
6
) building blocks were
then coupled to provide the corresponding amide (
29
) in all possible stereochemical
combinations. Intramolecular cyclizations (the pair phase) utilized the RCM macro-
cyclization reaction. Using this strategy, the complete stereochemical matrix of RCM
compounds was created in a modular fashion, lending itself to the generation of
powerful SSAR. The original RCM library, a subset of which was included in the
informer set, used solid-phase chemistry to create a library of 14,440 macrocycles.
Compound
30
contains a masked aniline, a protected secondary amine moiety, and a
protected alcohol, all excellent functionality for medicinal chemistry investigation.