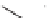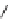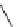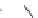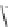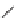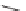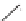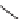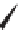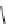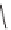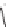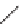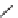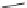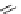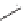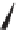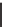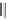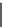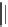Biomedical Engineering Reference
In-Depth Information
O
O
OH
O
Ph
R
2
R
2
O
(i)
(ii)
N
N
N
R
1
R
1
R
5
H
NH
2
NN
R
4
R
3
O
O
OH
OH
EtO
2
C
EtO
2
C
N
N
N
O
R
5
R
1
R
1
N
N
H
NH
2
R
4
Cl
Cl
(iii)
Cl
Cl
S
S
O
O
OH
O
EtO
N
HO
HO
N
N
H
NH
2
NO
2
NO
2
H
H
N
emmacin
gemmacin
gemmacin-B
FIGURE 1.6
Examples of compounds and scaffolds that exhibited anti-MRSA activity,
including (i) the four nitrogen heterocycle scaffolds from the library of Wyatt et al. [46];
(ii) active compounds from the library of Thomas et al. [47]; (iii) emmacin, (
−
)-gemmacin,
and (
±
)-gemmacin B.
showed increased efficacy in restricting bacterial growth [51]. Assays for common
antibacterial modes of action were performed (such as DHFR reductase inhibition,
protein synthesis, and ATP synthesis decoupling), but gemmacin proved inactive
in all of these assays. However, gemmacin did show activity in an assay to test
for the generation of reactive oxygen species, which suggests that gemmacin (and
gemmacin B) may act as bacterial cell-membrane disruptors [51]. The discovery
of these two antibacterial compounds, both of which represent a novel structural
class (or subclass), illustrates the power of the DOS approach for the discovery
of novel bioactive species. Figure 1.6 shows an overview of the structures of the
antibacterial compounds produced by the two DOS libraries, and the MIC
50
values of
the most active compounds against MSSA and two strains of MRSA are reported in
Table 1.1.
1.8.2 Use of Densely Functionalized Molecules
A recent example of the use of a reagent-based pathway to generate diversity
from densely functionalized molecules can be found in the work of Schreiber's