Biomedical Engineering Reference
In-Depth Information
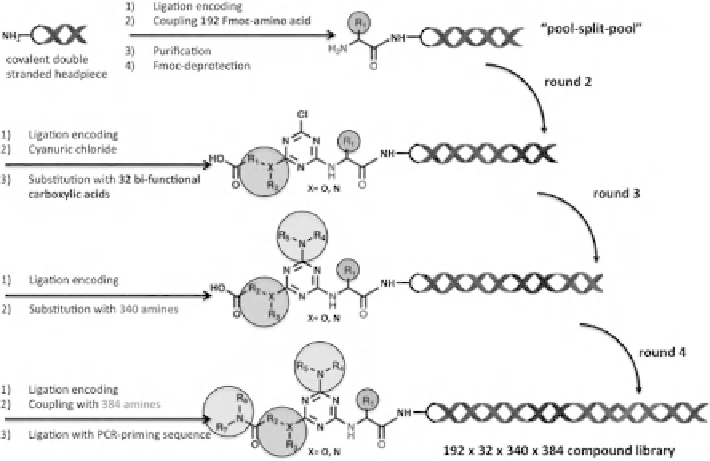
FIGURE 11.10
Praecis/GSK triazine-based DNA-conjugate library containing over 10
8
compounds. Pool-split-pool assembling of four different sets of building blocks and corre-
sponding DNA tags on a triazine scaffold yielded a DNA-encoded library containing nearly
1 billion compounds (192
×
32
×
340
×
384 members). A covalent double-stranded oligonu-
cleotide was used as a headpiece to shelter the DNA from chemical lesions during library
synthesis.
were stepwise substituted with a small set of 32 bifunctional carboxylic acids and
with 340 amines, respectively, in two consecutive rounds of split-pool-split synthesis.
Eventually, the carboxylic function installed in round 2 was activated and reacted with
additional 384 amines, to yield the final library containing nearly 1 billion compounds
(Figure 11.10) [58].
Selection and high-throughput-sequencing decoding of the library against p38
MAPK (p38 mitogen-activated protein kinase) revealed a cluster of conserved trisyn-
thon compounds to be preferentially enriched showing no preference for the identity
of the amine introduced in cycle 3 [58]. Resynthesis and structure analysis of repre-
sentative compounds from this family led to the identification of structurally related
triazine compounds (as potential side products of the library synthesis), with EC
50
in
the low-nanomolar range [58]. Notably, the crystal structure of one compound bound
to p38 MAPK displayed the carboxamide group, used initially to link the oligonu-
cleotide tag, pointing toward the solvent, thus not actively contributing to binding
with the target protein [58].
Despite the promising results reported by Praecis/GSK, the real efficacy of larger
chemical libraries, containing more than 10
6
compounds, is currently under debate
due to a number of technical limitations, including the limited purity of the library,