Biomedical Engineering Reference
In-Depth Information
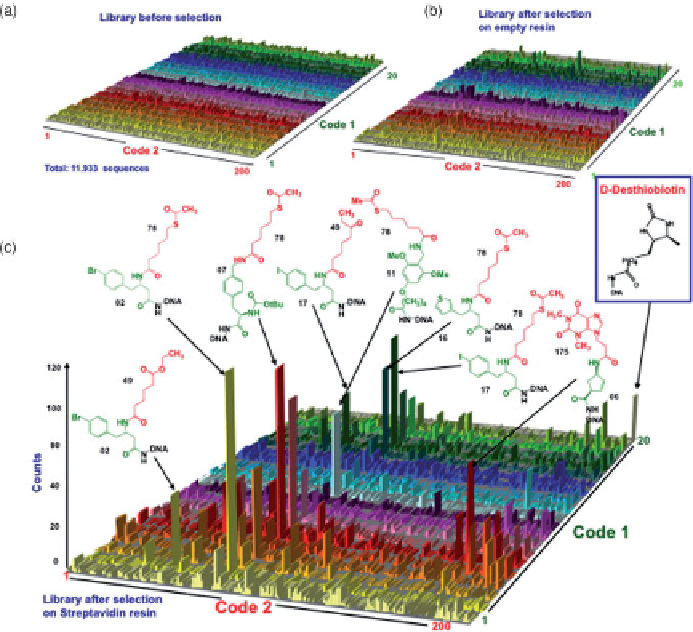
FIGURE 11.8
High-throughput-sequencing results before and after selection against
streptavidin-coated Sepharose resin. (a-c) Histogram plots represent the 4000 library mem-
bers in the
x-y
plane and the corresponding sequence counts on the
z
-axis. (a,b) Sequencing
before selection and after selection on an empty resin showed equally distributed frequencies
of library members; (c) streptavidin selection results exhibited a preferential enrichment of
D-desthiobiotin (known streptavidin binder with nanomolar affinity spiked into the library
prior to selection) and of additional structurally related compounds. The chemical structures
of some of the most relevant straptavidin binders are indicated together with the library iden-
tification number. Building blocks used in the two synthetic steps are highlighted in green
and red, respectively. Molecules identified with at least 30 counts exhibited after resynthesis
preferential binding toward streptavidin, (
K
D
values ranging between 350 nM and 11
M). In
contrast, compounds tested with fewer than 10 counts did not exhibit appreciable binding to
streptavidin (
K
D
>
50
M). (From [54]; copyright
C
2008 National Academy of Sciences,
U.S.A. (
See insert for color representation of the figure.
)
Speculating that double-stranded oligonucleotide would be able to shelter the DNA
from potential chemical lesions during library synthesis, a short covalently linked
DNA duplex was chosen as a headpiece [58]. Following acylation and deprotection
of 192 Fmoc amino acids onto the initial scaffold, a triazine ring was introduced using
cyanuric chloride. Subsequently, the remaining chlorines of the triazine headpiece