Biomedical Engineering Reference
In-Depth Information
PS2
6
4
2
-4
0
-2
2
4
0
6
PS1
8
2
4
6
PS3
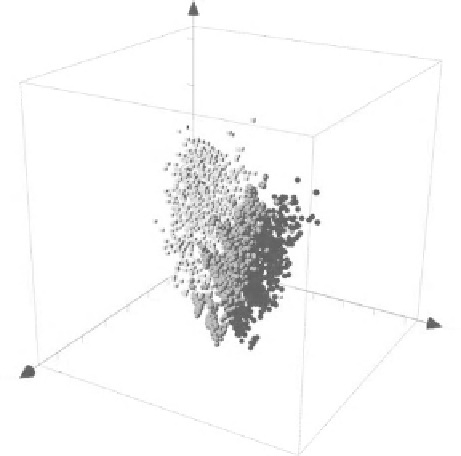
FIGURE 10.3
Visual representation of the chemical space of combinatorial libraries with
the core scaffolds of
1170
(dark gray),
1171
(light gray) in Figure 10.2a, and a set of approved
drugs (light gray). The plot was generated using ChemGPS-NP prediction scores calculated
using the online tool ChemGPS-NPWeb. Of note, the combinatorial libraries obtained with the
LOL approach occupy different regions of the chemical space.
Also using the six properties described above, the medicinally relevant chemical
space of 2477 natural products from a commercial vendor was recently compared to
5963 synthetic compounds from academic sources using methods such as DOS, and
6152 synthetic compounds from a commercial vendor (as representative of common
screening collections). The result of this comparison shows that the DOS compounds
considered in that study are heavier and more lipophilic than the natural products or
the synthetic commercial compounds [47].
Figure 10.3 shows a visual representation of the property-based chemical space
of two combinatorial libraries obtained using an LOL approach and a collection of
approved drugs. The visual representation was obtained using the recently developed
Web-based public tool ChemGPS-NPWeb [73,74]. ChemGPS-NP [73,75] is a PCA-
based global chemical positioning system [76] tuned for exploration of biologically
relevant chemical space. The first four dimensions of the ChemGPS-NP map capture
77% of data variance. The first dimension (principal component 1, PC1) represents
size, shape, and polarizability (the main contribution is size); PC2 is associated with
aromatic and conjugation-related properties (the main influence is aromaticity); PC3
describes lipophilicity, polarity, and hydrogen-bond capacity (the major contribution
is lipophilicity); and PC4 expresses flexibility and rigidity. Chemical compounds can