Biomedical Engineering Reference
In-Depth Information
There also exists something of a hierarchy within these types of diversity that is
based on both synthetic ease and the relative perceived value of each type. Appendage
diversity is viewed as the easiest to achieve but is the least important when it comes to
producing functionally (biologically) diverse compounds, and it is widely accepted
that scaffold diversity is by far the most important and most difficult to achieve
[2,32,33]. For this reason there are many published examples of DOS that focus
almost entirely on producing diverse molecular skeletons [31,34].
Scaffold diversity is considered the most important diversity element because
biomacromolecules are (on a molecular scale) large three-dimensional environments
with more or less defined binding regions, pockets, and surfaces; as such, they will
interact only with molecules that have complementary three-dimensional structure
[13,35]. Therefore, it is the overall shape of a molecule that is the most important
factor in terms of determining its biological effects, and this is linked intrinsically
to the molecular scaffold or skeleton that the molecule possesses [36]. Libraries that
contain large numbers of distinct molecular scaffolds should then cover the widest
range of potential binding partners.
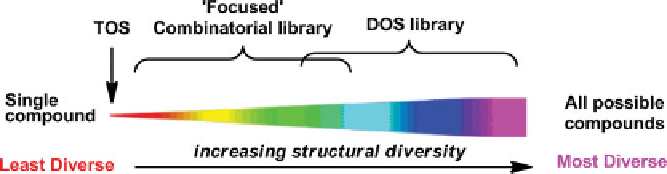
To provide a conceptually simple and easily interpretable comparison of the rel-
ative molecular diversity incorporated into different compound collections, Spandl
et al. suggested the consideration of molecular diversity as a spectrum [19]. At one
extreme of the spectrum is a single compound occupying a single point in chemical
space, and at the fartherest extreme are all possible compounds giving the maximum
chemical space coverage possible (Figure 1.2).
In this context, DOS aims to produce small-molecule libraries that occupy a
position toward the right-hand side of the spectrum. This qualitative representation
of molecular diversity on a sliding scale shows clearly the idea that DOS libraries
should be considerably more diverse than their traditional combinatorial counterparts;
however, it is not possible to use this spectrum to compare the relative diversity
of compound collections in any meaningful way. More quantitative assessment of
the relative diversity of compound collections can be achieved by looking at their
comparative molecular descriptors and using them computationally to generate a
visual representation of their positions in chemical space.
FIGURE 1.2
Molecular diversity spectrum: a representation of the relative degrees of molec-
ular diversity achieved using TOS, focused library synthesis, and DOS. (From [19], with
permission of The Royal Society of Chemistry.) (
See insert for color representation of the
figure
.)