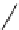Biomedical Engineering Reference
In-Depth Information
FIGURE 9.6
Chemical structure of artemisinin.
The efficiency of folding pathways may be increased if both the construction and
the folding of substrates may take place in a single reaction. The Nelson group has
exploited cascade chemistry [27], initiated by a three-component reaction, in the
synthesis of a range of alkaloid-like scaffolds (Scheme 9.10) [28]. The approach
could be used in combination with a second three-component reaction to control the
substitutional diversity of the final compounds.
The reaction between secondary amines
66
, carbonyl compounds
67
, and tri-
azines
68
yielded a range of skeletally diverse alkaloid-like compounds. Condensa-
tion between the secondary amines
66
and the carbonyl compounds
67
is believed to
generate enamines
69
, which can undergo an inverse-electron-demand Diels-Alder
reaction with the triazines
68
(
→
70
); expulsion of molecular nitrogen then yields
2-aza-dienes
71
, which are substrates for a subsequent folding pathway. The sub-
stituents in
71
determine the molecular scaffold that is ultimately obtained. Thus, in
the absence of a tethered dienophile, the 2-aza diene
71
is itself the product of the
reaction. However, with an appended dienophile in either the R
2
or the R
3
substituent,
a subsequent intramolecular Diels-Alder reaction can yield, for example, either
72
[with R
2
(
E
)-octa-3-enyl] or
75
(with R
3
allyl). Finally, with R
3
allyl, and
a pendant nucleophile in R
2
, intramolecular Diels-Alder reaction and subsequent
cyclization are possible (
=
=
=
73
).
In many cases, the three-component cascade reaction could be followed by a
second three-component reaction: a Joullie-Ugi reaction. Some selected examples of
the alkaloid-like products that were accessible using the approach are shown. Thus,
after the folding pathway, some of the products, such as the 2-aza diene
74
and
the cyclic imines
72
and
75
, were reacted with an isocyanide and a carboxylic acid
to give final products (such as
76
to
78
). By contrast, with an appropriate pendant
nucleophile in place (such as the NHCbz group in
73
), a cyclization terminated the
three-component cascade reaction. In total, 43 final products were prepared, which
were in general derived from five separate components. The products were based on
28 distinct graph-node-level frameworks;
†
the high scaffold diversity of the products
stemmed both from rings found in the individual components and from the power of
the folding pathway.
→
†
Frameworks defined at the graph-node level describe the connectivity and type of atoms (but not the bond
types between atoms).