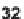Biomedical Engineering Reference
In-Depth Information
SCHEME 9.6
Overview of a Au-catalysed approach to the synthesis of skeletally diverse,
bicyclic heterocycles. X
=
OorCH
2
.
octanoate dimer in benzene at 50
◦
C. Presumably, generation of a carbonyl ylid was
followed by intramolecular 1,3-dipolar cycloaddition with the indole ring to give the
alternative polycyclic skeletons,
24
to
26
.
Yang et al. have developed a one-pot gold-catalyzed cascade to prepare skeletally
diverse alkaloid-like small molecules (Scheme 9.6) [24]. Initially, Au(I)-catalyzed
cyclization of alkynyl carboxylic acids
27
gave the corresponding cyclic enol ethers
28
. Subsequently, attack of amine nucleophiles
29
on the cyclic enol ether generated
ketoamides
30
. In other words, combination of the alkynyl carboxylic acid
27
and the
amine
29
dictated the structure of the cyclization precursor
30
. Under these reaction
conditions, the cyclization precursor was also converted into an
N
-acyl iminium
ion
31
, which was then trapped by a tethered nucleophile (
32
). The scope of
the methodology is summarized in Scheme 9.7. Using combinations of the alkynyl
carboxylic acids (e.g.,
33
or
34
) and the pyrrole- and indole-tethered amines (e.g.,
35
or
36
), a remarkable range of alternative alkaloid-like scaffolds was prepared (e.g.,
37
to
42
).
Dandapani et al. used radical cyclization chemistry to generate skeletally diverse
products (Scheme 9.8) [25]. The tetrahydropyridine substrates
43
to
45
were prepared
by stereoselective alkylation with a range of alkyl, alkenyl, and alkynyl halides. The
skeletons of the products
46
to
48
were preencoded by the location of the bromine
atoms and the unsaturated groups in the substrates
43
to
45
. The folding processes
were triggered by treatment of the tetrahydropyridines
43
to
45
with an appropriate
tin or silicon hydride and a substoichiometric amount of AIBN at 80
◦
C. Abstraction
of a strategically positioned bromine atom and cyclization yielded a range of distinct
polycyclic alkaloid-like frameworks (e.g.,
46
to
48
). Particularly high levels of skeletal
→