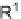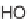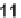Biomedical Engineering Reference
In-Depth Information
SCHEME 9.2
Illustrative syntheses of diverse molecules possessing a benzopyran core:
(a)
9
, (i) ArB(OH)
2
, 10 mol% Pd(PPh
3
)
4
,Na
2
CO
3
, 1,4-dioxane/H
2
O, (ii) HF-pyridine, THF;
(b)
10
, (i) ethynylmagnesium bromide, 10 mol% Pd(PPh
3
)
4
, ZnCl
2
, 1,4-dioxane, (ii) R
2
-N
3
,
BrCu(PPh
3
)
3
(20%), DIPEA, toluene, (iii) HF-pyridine; (c)
11
, (i) tributylvinyltin, Pd(PPh
3
)
4
(10%), 1,4-dioxane, (ii)
N
-phenylmaleimide, toluene; (d)
12
, (i) HF-pyridine, (ii) H
2
, 10%
Pd/C, MeOH-THF; (e)
13
, (i) DDQ, 1,4-dioxane, (ii) HF-pyridine. R- denotes a solid support.
Wilk et al. have prepared a library based on the scaffold of the alkaloid, macroline
(Figure 9.4) [21]. Initially, the immobilized tryptophan derivative,
14
, was reduc-
tively aminated, and then subjected to a Pictet-Spengler reaction, to yield the trans-
configured products
16
(Scheme 9.3). A Dieckmann cyclization was then used to
prepare the bridged cycloocta[
b
]indole ring system found in macroline, thereby