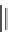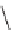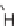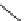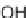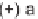Biomedical Engineering Reference
In-Depth Information
FIGURE 9.2
Chemical structure of secramine.
(
→
2
) was followed by conjugate addition of a thiol to the
,
-unsaturated ketone
(
4
). Finally, the ketones
4
were converted into imines or oxime ethers
5
. Following release from solid support,
the successful formation of 2527 of 2946 possible compounds was confirmed by
mass spectrometry. A cell-based phenotypic assay was used to identify secramine
6
(Figure 9.2) as a potent inhibitor of protein trafficking from the Golgi apparatus to the
plasma membrane. The discovery of secramine emphasizes the validity of exploiting
natural product scaffolds in the discovery of small molecules with novel functions;
secramine was inspired by galanthamine but has an unrelated biological function.
Oh et al. have reported the diversity-oriented synthesis of a library that was inspired
by benzopyran-containing natural products (see Figure 9.3 for examples of such
natural products) [16]. The vinyl triflates
8
, prepared from the corresponding ketones
7
, were the key intermediates in the synthesis (Scheme 9.2). The final compounds
9
were prepared by Suzuki coupling, followed by release from the solid support.
Alternatively, ethynylation, followed by a Cu-catalyzed “click” reaction, yielded the
triazoles
10
. In a similar vein, Pd-catalyzed vinylation of the vinyl triflates
8
, followed
by a Diels-Alder reaction, yielded the adducts
11
; the adducts
11
were either reduced
(
→
3
), and functionalization of the secondary amine (
→
→
→
13
) to yield final compounds with related scaffolds. Overall,
the approach was used to prepare a 434-member polyheterocyclic benzopyran library.
Nicolaou has described the synthesis of a member library of about 10,000 natural
product-like benzopyran derivatives using a distinct synthetic approach [17]. Other
libraries based on natural product-like scaffolds have exploited multicomponent aza-
Diels-Alder chemistry [18], cyclizations of products of multicomponent reactions
[19], and acetal formation [20].
12
) or aromatized (
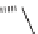
FIGURE 9.3
Examples of natural products with scaffolds related to benzopyrans.