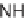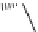Biomedical Engineering Reference
In-Depth Information
SCHEME 9.1
Pelish et al.'s Synthesis of galanthamine-like molecules with high appendage
diversity: (a) R
1
OH, PPh
3
, DIAD, THF, 0
◦
C; (b) R
2
SH, 2,6-lutidine,
n
-BuLi, THF, 0
→
40
◦
C;
(c) (i) R
3
CHO, AcOH, MeOH-THF; (ii) NaBH
3
CN, MeOH or R
3
COCl, 2,6-lutidine, CH
2
Cl
2
or R
3
NCO, CH
2
Cl
2
; (d) R
4
NH
2
, AcOH, MeOH-CH
2
Cl
2
.
[11c]. Such scaffold trees can facilitate navigation of the chemical space that is rel-
evant to a particular biological function; specifically, analysis of such trees can help
identify related, but previously unexplored, scaffolds that are worthy of investigation
[11a and b].
†
Scaffolds found in natural products have provided a direct inspiration in the design
of small-molecule libraries. Pelish et al. prepared a substitutionally diverse library
that was based on the scaffold of the alkaloid, galanthamine [15]. The scaffold
1
was prepared in five steps from a solid-supported tyrosine derivative. A sequence
of reactions was then used to vary four of the appendages (R
1
to R
4
) in the final
compounds (Scheme 9.1). Functionalization of the phenol using a Mitsunobu reaction
†
Scaffold Hunter is a software package that has been developed to facilitate the navigation of hierarchical
scaffold trees [11a and b].