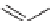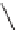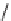Biomedical Engineering Reference
In-Depth Information
FIGURE 9.1
Examples of natural products exploited in chemical genetics.
does not inform DOS to the extent that compounds with novel functions are not
identified. In some cases, the scaffold (or scaffolds) represented within a DOS library
closely resemble those found in natural products. Inspiration from such scaffolds is,
however, valuable because ligands based on similar natural product scaffolds have
been shown to bind selectively to different ligand binding sites with similar folds [10].
In other examples, the relationship to natural products is much looser, with just some
of the broad structural features of a natural product family being represented. Given
that natural products necessarily have the structural features needed to recognize
biological macromolecules, this level of inspiration is appropriate; indeed, it can
allow the identification of ligands with functions that natural products have not yet
been found to possess.
Although in this chapter we focus on the underpinning synthetic strategy, examples
of valuable small molecules discovered using natural product-like libraries will be
provided. In each scheme, generic structures for the synthetic intermediates are
shown, generally with illustrative examples of specific final compounds. In Section 9.2
we focus on the synthesis of libraries that are inspired directly by a single (or a limited
range of) natural products. A key challenge in the field has, however, been to vary
the scaffolds of natural product-like molecules more widely. Some general strategies
have been developed for the synthesis of skeletally diverse small molecules, including
folding pathways (Section 9.3), branching pathways (Section 9.4), and oligomer-
based approaches (Section 9.5).
9.2 LIBRARIES INSPIRED BY NATURAL PRODUCT SCAFFOLDS
Small-molecule scaffolds play a key role in guiding chemists' navigation of bio-
logically relevant chemical space [11]. It has been proposed that biologically active
small molecules may be focused on specific subfractions of chemical space [12].
Indeed, the field of biology-oriented synthesis (BIOS) [10d,13] seeks to target such
“bioactivity islands” by designing libraries around scaffolds that have been validated
biologically. Some drug scaffolds have been described as privileged [14] because
representative compounds bind to different protein targets. The scaffolds found in
natural products have been mapped comprehensively using hierarchical scaffold trees