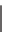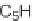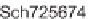Biomedical Engineering Reference
In-Depth Information
SCHEME 8.14
Diastereomeric library of the natural macrolactone Sch727654 using a flu-
orous mixture synthesis strategy.
macrocyclization strategies: S
N
Ar, Huisgen [3
2] cycloaddition (click), RCM, and
macrolactamization [90]. These scaffolds combine some of the features of natural
products and small molecules, such as contiguous chiral centers, endocyclic amides,
or secondary alcohols. Libraries in the order of 15,000 members were produced using
this approach, which have reportedly yielded leads on two targets. On the one hand,
macrocycle
85
was found to be a low-micromolar inhibitor of histone deacetylases 1,
2, and 3 [90a]. On the other hand, macrocycle
86
inhibited the growth of the malaria
parasite
Plasmodium falciparum
, with an IC
50
value of 0.51 nM [91]. Although the
target has not been identified, the compound displayed no toxicity toward erythro-
cytes.
+
8.5.4 Multicomponent Macrocyclization
Multicomponent reactions have been used extensively to generate diverse libraries
of small molecules (see also Chapter 2). They have also been applied to the