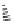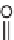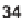Biomedical Engineering Reference
In-Depth Information
FIGURE 8.4
Conformationally constrainted macrocyclic tripeptide mimetics.
enzyme conformation at the binding site or at remote sites, a reciprocal phenomenon
termed
induced fit
. Reciprocal conformational adjustment of both the enzyme and
the inhibitor is common, which makes the prediction of enzyme/inhibitor geome-
try more difficult. Reid et al. reported the use of conformational constraints in the
form of macrocyclic tripeptide mimetics, with the goal of minimizing the confor-
mational rearrangement of the inhibitor upon binding (Figure 8.4) [54]. Macrocyclic
templates
33
and
34
were designed to achieve this goal, based on the analysis of
x-ray structures of protease-bond peptide inhibitors.
33
and
34
mimic the extended
conformation of bound linear inhibitors, giving them an entropic advantage upon
binding. Additionally, these macrocycles are more stable to proteolytic degradation
and generally possess higher bioavailability then that of their linear congeners [55].
Accordingly, the authors produced a collection of N- and C-terminal macrocycles
(
33
and
34
, Figure 8.4). Variations of R groups led to the identification of low-
nanomolar HIV-1 protease inhibitors. Crystallographic analysis of protease/inhibitor
complexes revealed a high level of conservation in residues surrounding the inhibitors
and among contacts between inhibitors and enzyme. This suggests that induced fit is
reduced among this series of macrocyclic HIV-1 inhibitors and opens new avenues
for inhibitors of additional proteases as well.
Park and Burgess reported several classes of macrocyclic peptidomimetics
designed to mimic protein
-turns [56]. Starting from solid-supported precursor
35
(Scheme 8.7), the macrocyclization relied on a key S
N
Ar reaction to yield macro-
cycle
36
. Oxygen, nitrogen, and sulfur nucleophiles all performed well in the S
N
Ar
reaction, which was facilitated by the presence of the adjacent iodine atom [57]
and could subsequently be diversified by cross-coupling. The same group reported
the synthesis of semipeptoid analogs using an analogous approach [58]. As shown
in Scheme 8.7, starting from solid-supported precursor
37
, macrocyclic semipep-
toids were obtained by S
N
Ar to provide macrocycles
38
. Similarly, precursor
39
was
cyclized by S
N
2 on the benzylic bromide to provide, after cleavage from the resin,
macrocycles
40
. Molecular modeling and conformational analysis by solution NMR
indicated that these compounds adopted the desired
-turn-like conformations. This
class of macrocycles led to the identification of TrkA-dependent NGF mimics and
TrkC-dependent NT3 mimics
41
and
42
[59].
A related approach was reported by Jefferson, Swayze, and co-workers involving
a combinatorial library of about 12,000 macrocycles based on scaffold
43
, which