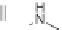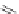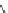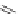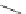Biomedical Engineering Reference
In-Depth Information
technology was applied in microfluidics settings [42], and led to the design of iso-
cyanide
13
, which enabled the synthesis of solvatochromic, conformation-responsive
macrocycles and ionophores [43]. Subsequently, they synthesized a macrocycle capa-
ble of imaging mitochondria selectively [43]. Finally, the same authors demonstrated
that fluorescently labeled 18- and 21-membered macrocycles
14
(
c
-PRGDA) and
15
(
c
-PRGDAA) were both able to bind the
v
3
integrin receptor in a cell adhesion
assay with affinities of 4.2 and 11.1
M, respectively, as opposed to scrambled analog
16
(
c
-PRDGA) corroborating computational predictions [44]. This technology plat-
form holds promise to support conformational fine-tuning in subsequent optimization
[45].
8.3.3 DNA-, RNA-, and Phage-Templated Synthesis of Peptidic Macrocycles
The translation of nucleic acids into synthetic compounds is a recent achievement that
has been pioneered largely by Liu and co-workers [46]. The authors have exploited
DNA-templated chemistry in several directions, providing an efficient solution to the
problem of broad diversity generation in massive libraries while exploiting one of
the most robust encoding approaches available. This technology was applied to the
synthesis of macrocyclic peptide-like structures (Scheme 8.5) [47]. Using a set of
DNA codons able to support large library synthesis, the group constructed a library
of 14,000 macrocycles of general structure
28
. Macrocyclization hinges around a
biocompatible version of the Wittig reaction, which transformed DNA-linked pre-
cursor
27
into DNA-linked macrocycle
28
. Of particular interest, reactions were run
at nanomolar concentrations, which minimized dimerization and building block con-
sumption. In this application, DNA templating provides voth high effective molarities
and high dilution ideal for monomer macrocyclization. Using this approach, libraries
were produced and tested as mixtures in solution. Upon isolation of an active product,
SCHEME 8.5
DNA-templated
chemistry
applied
to
the
synthesis
of
peptide-like
macrocycles.