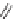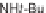Biomedical Engineering Reference
In-Depth Information
SCHEME 8.3
Multicomponent reaction using aziridine carboxaldehyde
11
as an amphoteric
reagent to achieve macrocycles.
8.2.2 Assessment of Diversity
Molecular diversity in the chemical space of macrocycles is a parameter that has not
been analyzed quantitatively, as opposed to extensive studies on small molecules [27].
Molecular diversity in macrocycles can be coarsely divided between the chemical
functionality displayed by the scaffold on the one hand, which will often be critical
in establishing direct interactions with a biological target, and on the other hand
the topological diversity that defines the overall shape and volume occupancy of the
molecule. These two parameters are intertwined with respect to target interactions
and PK-ADME profile. To better characterize diversity in macrocyclic natural prod-
ucts, Wessjohann, Frank, Lachance, and others have analyzed naturally occurring
macrocycles using data mining and cheminformatics [10,28]. This was done from a
structural point of view based on molecular weight distribution (up to 2559 g/mol),
ring size (13- to 72-membered) and the occurrence of common substructures, and
from a production point of view taking into account biosynthetic pathways (polyke-
tide pathways, isoprenoid metabolism, peptide biosynthesis and sugar condensation)
[10,13a]. Macrocycles, which constitute about 3% of all natural products, possess
very complex and diversified structures, as well as high levels of biological activi-
ties. Ranging from FK-506 (
17
, Figure 8.1) to erythromycin A (
18
), epothilone B
(
19
), vancomycin (
20
), and amphotericin B (
21
), the authors observed the predom-
inance of 14-membered rings and the frequent assembly, in the same molecule, of
an apolar, hydrophobic side and a polar, hydrophilic side, or the presence of at least
one small heterocycle. The diversity in naturally occurring macrocycles is further