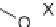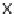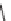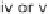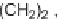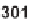Biomedical Engineering Reference
In-Depth Information
SCHEME 7.41
Synthesis of cyclobutanes and ring expansion to lactone and lactam deriva-
tives. Reagents and conditions: (i) THF, NaHCO
3
(aq.); (ii) TMSOK, MeOH, DCM or pyrro-
lidine; (iii) LiBH
4
, MeOH, THF; (iv) MeMgCl, THF,
−
78
◦
C
→−
10
◦
C; (v) Me
4
NBH(OAc)
3
,
DCM; (vi)
m
CPBA, DCM; (vii)
O
-mesitylenesulfonylhydroxylamine, DCM, rt.
The polymer-supported cyclobutanone was cleaved from the resin directly by treat-
ment with TMSOK to afford liberated cyclobutanone
299
. Cyclobutane with hydroxyl
group
300
was prepared via two routes. The first route involved nucleophilic addition
with Grignard reagent and subsequent cleavage from the resin. The second involved
reduction with LiBH
4
to yield tertiary alcohol
300
(R
4
=
Me) and secondary alcohol
300
(R
4
H), respectively. Alternatively, iminium ions
297
were converted into
amino derivatives
303
. In addition to four-membered ring hydrocarbons, ring expan-
sion to five-membered lactons and lactams was also performed. Baeyer-Villiger ring
expansion of ketones
298
afforded
=
-lactones
301
, while Beckmann rearrangement
yielded
-lactams
302
.
The next example outlines the synthesis of a pyranone-derived scaffold. Polymer-
supported furyl alcohols
304
were oxidized to 2
H
-pyran-3(6
H
)-ones
305
, having
many reactive sites and functionalities and thus amenable to further transformation
into diverse pharmacologically important heterocyclic compounds by selective pair-
ing (Scheme 7.42) [4].
The key precursor
305
was converted into carbamate
306
that was either
treated with alcohols to give ethers
307
or afforded oxazolediones
308
under basic