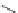Biomedical Engineering Reference
In-Depth Information
R
3
O
R
1
N
SO
2
O
R
2
NO
2
290
i
iv
R
3
O
O
O
R
3
R
3
R
1
R
1
R
1
N
ii
N
H
N
SO
2
O
N
O
N
O
R
2
O
O
R
2
R
2
NH
2
292
291
295
iii
ii
R
3
= OEt
R
3
O
R
3
O
R
1
H
O
2
S
N
ii
O
N
N
R
2
R
2
R
2
R
1
R
1
N
N
H
H
O
O
O
293
294
296
SCHEME 7.40
2
H
-Indazole 1-oxides-ring expansion to quinazolines and to 2,3-
dihydrobenzo[
f
][1,2,5]thiadiazepine 1,1-dioxides. Reagents and conditions: (i) 0.2 M DBU,
DMF, rt, 30 min; (ii) 50% TFA/DCM, rt, 1 h; (iii) 0.1 M to 0.2 M DBU, DMF, rt, 10 min to
16 h; (iv) SnCl
2
·
2H
2
O, DIEA, DMF, rt, on.
to rearrangement involving 2
H
-indazole 1-oxide core opening followed by quina-
zoline
293
ring closure.
N
-Alkyl-2-nitro-
N
-(2-oxo-2-arylethyl)benzenesulfonamides
290
can also serve for the synthesis of (6
7)-membered heterocycle
296
. However,
this route does not represent a scaffold hopping process.
+
-lactams, and cyclobutane derivatives were synthesized from Mer-
rifield resin-supported cyclobutanone precursors
298
[61,79]. [2
-Lactones,
+
2] Cycloaddition
was used to prepare cyclobutanone iminium species
297
that was hydrolyzed to the
ketones
298
and further subjected to various reaction routes, leading to either four-
or five-membered rings (Scheme 7.41).