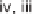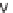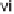Biomedical Engineering Reference
In-Depth Information
SCHEME 7.36
Synthesis of a library of bis-heterocyclic compounds. Reagents and con-
ditions: (i) SnCl
2
·
2H
2
O, DIEA, DMF, rt, 2 h to on; (ii) Fmoc-amino acid, DIC, THF (for
temperature and time, see original paper); (iii) 50% piperidine/DMF, rt, 30 min; (iv) Fmoc-
NCS, DCM, rt, 1 h; (v) haloketone, DCM, rt, on; (vi) 50% TFA/DCM, rt, 30 min; (vii) AcOH,
60
◦
C (for reaction time, see original paper), then 5% NaOH/MeOH, 60
◦
C, on (when amino
alcohols were used in the first combinatorial step).
couple phase involves intermolecular coupling reactions. At this stage building blocks
are joined in all stereochemical combinations and yield functionalized molecules
ready to enter into the last stage, the pair phase. In this last step, intramolecular
coupling reactions (functional group-pairing reactions) between appendages within
the molecule lead to diverse scaffolds [2]. The simplest illustrious example of the
B/C/P strategy is the synthesis of cyclic peptides using L- and D-amino acids.
The synthetic strategies of several DOSs that fall into more than one category
were described in previous sections. For example, in 2006, two years before the
B/C/P pathway was introduced [2], Mitchell and Shaw described the synthesis of a
diverse library of polycyclic lactams that can be regarded as a B/C/P approach [68].
The synthesis began with the build step, which involved the preparation of polymer-
supported methoxy-substituted oxazole
264
and of various aromatic aldehydes as
well as benzyl halides decorated with azide in the ortho position (both prepared in
solution) (Scheme 7.38). In the next step, the couple phase [step (i)], enantioselective