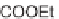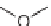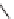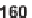Biomedical Engineering Reference
In-Depth Information
SCHEME 7.22
Conversion of propenoate into four different heterocycles. Reagents and
conditions: (i) Bredereck's reagent, toluene, 100
◦
C; (ii) pyridineacetic acid derivatives,
AcOH, 90
◦
C; (iii) 50% TFA/DCM, rt; (iv) 2-aminopyridine derivatives, AcOH, 90
◦
C; (v)
3-aminopyridazine, AcOH, 90
◦
C; (vi) 2-aminothiazole, AcOH, 90
◦
C.
Other libraries of heterocyclic scaffolds were achieved from the key intermediate,
resin-bound propenoate
161
prepared by reaction of Wang resin with ethyl isocyana-
toacetate and subsequent treatment with Bredereck's reagent (Scheme 7.22) [45]. The
intermediate
161
was then cyclized with different nucleophiles such as pyridineacetic
acid derivatives [step (ii), Scheme 7.22], 2-aminopyridine derivatives [step (iv)], 3-
aminopyridazine [step (v)], and 2-aminothiazole [step (vi)] to form, after the cleavage
from the resin, bicyclic heterocyclic scaffolds
162
to
165
.
Hetero-Diels-Alder reaction of the diene-containing natural product piperine with
polymer-supported acyl- and arylnitroso dienophiles was applied for synthesis of
structurally diverse heterocycles [46]. An attractive feature of the hetero-Diels-Alder
reaction of diene-containing natural products with acyl- and arylnitroso derivatives
is its potential to generate versatile cycloadducts (evolvable scaffolds) and provided
opportunities for “Modular enhancement of nature's diversity” (MEND) [47]. Con-
version of cycloadduct intermediate
166
into four diverse products is shown in
Scheme 7.23. The synthesis utilized diisopropylsilyl linker
I
and Rink linker
II
.
Cleavage of the precursor
166
from the resin with 50% TFA in DCM led to 1-(2-
nitrophenyl)-1
H
-pyrrol-2-yl(piperidin-1-yl)methanone
167
, while reduction of nitro
group prior to the cleavage from the solid support afforded 1-(benzo[
d
][1,3]dioxol-
5-yl)pyrrolo[1,2-
a
]quinoxalin-4(5
H
)-one
168
. Reduction of the nitro group to an
amino group and subsequent cleavage from the silicon linker opened access