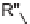Biomedical Engineering Reference
In-Depth Information
FIGURE 7.1
Generic structures of 10 skeletons prepared from vanillin-based precursors.
dihydrotriazepinone
145
, was prepared from bromoacylamino ketone
142
(R
4
=
Br)
by treatment with BnNHNH
2
·
HCl and spontaneous cyclization during acid-mediated
cleavage from the resin. The fourth reaction pathway resulted in formation of mor-
pholinone
146
. BEMP-mediated cyclization of bromoacylamino ketones
142
(R
2
=
aryl, R
4
Br) afforded polymer-supported oxazinones that were released from the
resin by TFA in DCM in the presence of triethylsilane (TES) as a reducing agent to
afford morpholinones
146
.
=
-Acylamino ketones
142
were also utilized for synthesis
of imidazoles
147
. In this case,
-acylamino ketones
142
were cyclized in acetic
acid in the presence of AcONH
4
and yielded imidazole derivatives
147
. Treatment
of precursor
142
with Schwesinger base (BEMP) caused cyclization to lactam that
was subsequently released from the resin to get heterocycle
148
. The last heterocycle