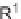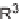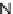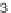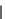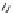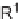Biomedical Engineering Reference
In-Depth Information
SCHEME 7.14
Transformation of
-ketosulfone into four diverse heterocycles. Reagents
and conditions: (i) LiCH
2
SOMe, 2,3-disubstituted oxirane; (ii) Jones reagent, acetone, 0
◦
C;
(iii)
N
(R
4
)-urea or
N
(R
4
)-thiourea; (iv) guanidine or benzamidine; (v)
o
-phenylenediamine.
polymer-supported sulfone,
84
(Scheme 7.14). Subsequent sulfone anion alkylation
with an epoxide led to formation of
-hydroxyl sulfone, which was oxidized to
-ketosulfone
85
. Finally, one-pot elimination-cyclization led to final compounds
pyrimidine-2-ones
86
(X
=
O), pyrimidine-2-thiones
86
(X
=
S), pyrimidines
87
,
and benzo[
b
][1,4]diazepines
88
, respectively.
Five various nitrogenous nonplanar heterocycles including five- to seven-
membered rings were prepared from solid-supported
N
-acylated amino acid amides
89
(Scheme 7.15) [34]. Two amides were reduced to afford the key precursor hav-
ing two secondary amino groups, polymer-supported ethylenediamines
90
. Further
reaction with different bifunctional reagents resulted in formation of heterocyclic
scaffolds with therapeutic and diagnostic potential. Treatment of diamine precursor
90
with carbonyldiimidazole, thiocarbonyldiimidazole, or cyanogen bromide yielded
imidazolidin-2-one
91
(X
=
O), imidazolidine-2-thione
91
(X
=
S), and imidazolidin-
2-imine
91
(X
NH), respectively. Reaction of precursor
90
with oxalyldiimidazole
led to formation of diketopiperazines
92
; further reduction afforded piperazines
93
.
Reaction with malonyl dichloride gave diazepinediones
94
, and finally, treatment
with carbonisocyanatidic chloride afforded triazinediones
95
.
Further, five- and six-membered bis-heterocyclic compounds were prepared from
resin-bound orthogonally protected lysine
96
(Scheme 7.16) [34,35]. The reac-
tion sequence was based on consequent selective cleavage of particular protecting
groups and acylation of liberated primary amino groups. Subsequently, all amidic
=