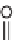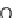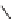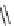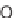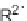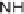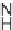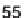Biomedical Engineering Reference
In-Depth Information
their isomers, such as 1
H
-pyrazoles, isoxazoles, and 1
H
-1,2,4-triazoles, were also
described [22-24]. Fused derivatives (i.e., benzimidazoles, benzoxazoles, and ben-
zothiazoles) have been prepared from Wang resin-bound esters
55
by condensation
under microwave irradiation [25].
The DOSs of various nitrogen- and oxygen-containing heterocyclic
scaffolds—furo[3,4-
d
]pyrimidines, pyrrolo[3,4-
d
]pyrimidines, and pyridazino[4,5-
d
]pyrimidines—were prepared on hydroxymethylpolystyrene resin [26]. The key
common intermediate
56
was prepared from resin-bound 4-chloroacetoacetate pre-
cursor
55
by three-component Biginelli-type condensations utilizing urea and various
aromatic aldehydes (Scheme 7.10). Precursor
56
afforded furo[3,4-
d
]pyrimidines
57
by cyclative cleavage. Pyrrolo[3,4-
d
]pyrimidines
59
were obtained by reaction
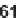
with primary amines followed by cyclative cleavage. Pyridazino[4,5-
d
]pyrimidine
derivatives
61
were achieved by substitution with hydrazines following cyclative
SCHEME 7.10
Synthesis of three various heterocycles embedded with 3,4-
dihydropyrimidin-2(1
H
)-one core skeleton by cyclative cleavage. Reagents and conditions:
(i) R
1
CHO (3 equiv), urea (3 equiv), dioxane, HCl (cat.), 70
◦
C, on, then conc. HCl, rt, 5 min;
(ii) DMF,
w (150 or 200
◦
C), 10 min; (iii) R
2
NH
2
(5 equiv), DMF, rt, 50 or 70
◦
C, on; (iv)
R
3
NHNH
2
(5 equiv), DMF, rt, 30 min.