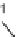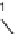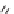Biomedical Engineering Reference
In-Depth Information
immobilized deliberately via an alkylsilyl linker through a three-carbon linker, which
served as a spacer, utilized a primary hydroxyl group for immobilization, and also
enabled printing the small-molecule library onto glass slides and conducting protein-
binding studies with small-molecule microarrays [20].
The Alloc group was cleaved from the aminoindoline precursor
44
, and the resin-
bound amine was acylated with BzCl. After Fmoc-protecting group removal, the
intermediate was coupled with acryloyl chloride to yield compound
45
. The key step
of this reaction sequence, the ring-closing metathesis, yielded, after cleavage from
the resin, the final tricyclic compound
46
.
Alternatively, the Fmoc-protecting group was removed from the resin-bound pre-
cursor
44
. Subsequent conversion of the amine to amide was followed by Alloc
removal. Liberated amine was subjected to reductive alkylation to yield secondary
amine. Acylation with acryloyl chloride led to intermediate
47
, which underwent
ring-closing metathesis, yielding compound
48
.
7.2.1.1.3 Heterocycles with More Heteroatoms
Several studies focused on solid-
phase synthesis of five-membered heterocyclic structures containing nitrogen, oxy-
gen, and sulfur heteroatoms. Synthesis of 1
H
-imidazoles, thiazoles, and oxazoles
was reported on polystyrene sodium sulfinate resin [21]. Resin-bound sulfinic acid
49
afforded precursor
50
by condensation with aldehyde and amide. Cleavages
of the products by one-pot elimination-cyclization reaction yielded three five-
membered heterocycles:
51
to
53
(Scheme 7.9). Polymer-supported syntheses of
SCHEME 7.9
Synthesis of three different five-membered heterocycles. Reagents and condi-
tions: (i) R
1
CHO, R
2
CONH
2
, TMSCl, MeCN/toluene, 80
◦
C; (ii) reagent
54
(20 mol%), TEA,
DCM, R
3
CHO; (iii) EtOH, R
4
NH
2
, AcOH, reflux; (iv) Lawesson's reagent; (v) PPh
3
,I
2
.