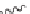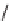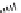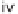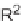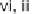Biomedical Engineering Reference
In-Depth Information
SCHEME 7.5
Synthesis of 2,5-dihydro-1
H
-pyrroles and their fused derivatives. Reagents
and conditions: (i) R
2
COCl, TEA, DCM, rt, 24 h; (ii) 30% H
2
O
2
, THF, rt, 1 h; (iii) R
2
NCO,
toluene, rt, 12 h; (iv) DBU, 80
◦
C, 6 h; (v) ClCH
2
COCl, TEA, DCM, rt, 24 h; (vi) R
2
NH
2
,
TEA, MeOH/THF, reflux, 10 h.
anxyolytic, and antipsychotic properties [15], while spirodiketopiperazines exhib-
ited antiproliferative and antiinflammatory activities [16].
The synthetic route started with a diastereomeric mixture of Merrifield resin-
bound amino ester
31
(Scheme 7.6). This precursor was reacted with either phenyl
isocyanate or Fmoc-amino acid. Reaction with phenyl isocyanate yielded derivative
32
, which was cyclized to final spirohydantoin
33
. Acylation with Fmoc-alanine
afforded intermediate
34
. Cleavage of a Fmoc-protecting group triggered spontaneous
cyclization to final spiro-2,5-diketopiperazines
35
and cleavage from the resin. After
evaluation of the scope and limitations of the synthetic route, two libraries of 56
spirohydantoins and 56 spiro-2,5-diketopiperazines were prepared in a combinatorial
manner.
Other work described a DOS of skeletally diverse alkaloid-like compounds
[17,18]. A precursor of the distinct products was the polymer-supported dihydroiso-
quinoline
38
together with dihydropyridine. Only the route involving the dihy-
droisoquinoline skeleton is outlined here. 7-Hydroxyisoquinoline was attached to
macrobeads to yield compound
36
, which was alkylated with
o
-bromobenzyl bro-
mide to afford iminium salt
37
(Scheme 7.7). Addition of vinyl magnesium bro-
mide yielded the target precursor dihydroisoquinoline
38
. It is worth noting that
polymer-supported dihydroisoquinolines (as well as dihydropyridines) were sta-
ble compared to those not attached to the solid support. This enamine was sub-
jected to different reactions; selective reduction with NaBH
3
CN in acidic conditions
led to 1,2,3,4-tetrahydroisoquinoline
39
. The second route involved reaction with