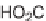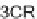Biomedical Engineering Reference
In-Depth Information
SCHEME 6.17
Classic Passerini-3CR approach to peptidomimetic macrocycles.
of hybrid systems as an entry toward a nonrepetitive type of macrocycle resembling
the structural diversity of those naturally occurring, which are usually endowed with
unique structural complexity. Specifically, the straightforward strategy was based on
multicomponent reactions to generate a collection of very large chimeric peptoid
macrocycles, specifically containing steroid moieties in this report.
Successively, Leon et al. reported additional examples of multicomponent macro-
cyclizations using both Staudinger and Passerini three-component reactions [39].
In a specific report, the versatility and wide scope of the macrocylization method-
ology were shown to achieve complex macrocyclic structures in one pot and in
a straightforward manner with multiple functional groups encompassing multiple
Staudinger-3CRs and Passerini-3CRs (Scheme 6.17).
A very nice entry to the synthesis of macrocyclic peptidomimetics accord-
ing to DOS principles was proposed recently by Isidro-Llobet et al. [40], who
reported the strategy for preparing amino acid-containing macrocycles based on the
build/couple/pair method introduced by Nielsen and Schreiber [41]. The study con-
sisted of the synthesis of a library of 14 structurally diverse and complex compounds
containing structurally diverse small molecules based around biologically relevant
macrocyclic peptidomimetic frameworks. All building blocks were prepared from
simple, commercially available amino acid derivatives. Specifically, azido-amine
building blocks,
-azido acids and alkyne-acid building blocks from phenylalanine
and lysine, respectively, were taken into account.
The build step focused on two types of chiral building blocks: the first containing
a free amine and an azide (“azido-amine” building blocks), and the second having
a carboxylic acid and an alkyne (“alkyne-acid” building blocks). The coupling step
of three of these building blocks via amide bond formation gave a range of tripep-
tide derivatives, thus providing the stereochemical diversity. The pair phase allowed
for scaffold diversity by accessing two cyclization steps in the formation of macro-
cycles containing either the triazole or both the triazole and diketopiperazine rings
(Scheme 6.18).