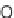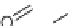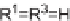Biomedical Engineering Reference
In-Depth Information
SCHEME 6.15
Stepwise and one-pot processes to address skeletal diversity around the
starting morpholine ring. (From [35], with permission; copyright
C
2010 Elsevier.)
time, which is the time interval required for a yeast cell to divide, or in cell growth at
the stationary phase, or both, were selected for further characterization [36].
Screening of the wild-type strain allowed for the selection of 21 molecules induc-
ing a phenotypic effect higher than 10% or other interesting effects (e.g., a decrease
in yeast fitness). In a second round of phenotype screening, eight molecules were
selected and tested for mitochondrial membrane potential activation and peroxisomal
proliferation. Both mitochondrial membrane activation and peroxisomal prolifera-
tion are indicators of the metabolic state of a cell, disclosing respiratory and fatty
acid metabolism, respectively, and ultimately resulting in the identification of new
chemotypes involved in mitochondria metabolism and respiration events.
6.5 MACROCYCLIC PEPTIDOMIMETIC SCAFFOLDS
The field of macrocycles in diversity-oriented synthesis is emerging, and it is a
very promising research area in addressing the need for peptidomimetic compounds
targeting extended protein-protein interactions (see Chapter 8 for a comprehensive
review of the topic). In a series of publications, Wessjohann and Ruijter described the
successful generation of macrocyclic peptide-based structures taking advantage of
multicomponent reactions to address both chemical diversity and macrocyclization
[37]. Specifically, they applied an expanded strategy using the potential of multicom-
ponent reactions not only for diversity generation, but also for the macrocyclization
reaction. This approach allowed for building a complex macrocycle in one step from
simple precursors, taking advantage of at least one bifunctional building block as the
main requirement.
In case of a single Ugi reaction forming the ring, two of the four R groups were
replaced by a tether joining two functionalities. This approach required three com-
ponents to achieve macrocyclization through a single Ugi reaction, one being an
unsymmetrically bifunctional building block (see Scheme 6.16). When employing
more than one Ugi reaction to construct macrocycles, several bifunctional build-
ing blocks were required to carry out multiple-multicomponent macrocyclizations.