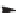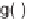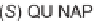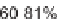Biomedical Engineering Reference
In-Depth Information
SCHEME 6.7
Enantioselective 1,3-dipolar cycloadditions of azomethine ylides with
electron-deficient olefins in the divergent pathways for the generation of highly substituted
proline analogs.
scaffolds [21]. Thus, in view of expanding the array of peptidomimetics according
to DOS principles, proline is a privileged template as the starting point for the con-
struction of skeletal diversity of complex scaffolds from the chiral pool or taking
advantage of asymmetric processes and the extensive amount of chemistry focusing
on the generation of pyrrolidine-based scaffolds.
Chen et al. described a procedure for the synthesis of highly substituted proline
analogs, taking advantage of the use of enantioselective 1,3-dipolar cycloadditions
of azomethine ylides with electron-deficient olefins in the divergent pathways of
diversity-oriented synthesis [22]. The stereospecificity of the reaction enabled stere-
ochemical diversification of up to four tetrahedral centers on pyrrolidine rings, using
a silver(I) acetate/QUINAP catalyst system as being most effective for the reaction
of azomethine ylides derived from benzaldehyde and several
-amino esters, with
3 mol% catalyst loading of various dipolarophiles (Scheme 6.7)
A very interesting entry to peptidomimetic scaffolds containing the proline ring
according to DOS principles was recently reported by Hung et al. [23]. In this paper,
a build/couple/pair (B/C/P) strategy was devised to achieve small fused bicyclic and
spirocyclic three-dimensional fragments consisting of nonaromatic, high-sp
3
content,
and chiral fragments. The pathways were constructed to generate small molecules
according to the
fragment rule of 3
[24], which is a structural criterion for molecular
compounds to limit the molecular weight to less than 300 g/mol and to allow for
a maximum of three hydrogen-bond donors or acceptors and a maximum of three
rotatable bonds.
The strategy was exemplified by selecting three proline analogs as the building
blocks and applying coupling reactions with alkene derivatives to access the final