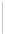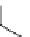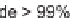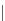Biomedical Engineering Reference
In-Depth Information
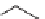
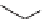
SCHEME 6.6
Synthetic approach to diastereoselective 2,5-disubstituted diverse morpho-
lines, piperazines, and thiomorpholines from
-amino acid derivatives and molecular iodine.
access to further skeletal diversity by extending the process to other unnatural amino
acid-based building blocks.
In a recent paper, Bera and Panda described the synthesis of disubstituted six-
membered ring heterocycles taking advantage of the chemistry of alkenes with I
2
[19]. This simple and powerful synthetic route provided access to diastereoselective
2,5-disubstituted diverse morpholines, piperazines, and thiomorpholine starting from
commercially available
-amino acid-derived synthetic intermediates (Scheme 6.6).
The key step involved iodine-mediated cyclization under mild reaction conditions to
give six-membered ring heterocycles. Such compounds may be elaborated further
in several ways, such as by nucleophilic substitution on the rings as well as by
incorporating a substituent at the 5-position from amino acids constituents.
6.4.2 Scaffolds Containing the Pyrrolidine Ring
Proline has a special place as a template for the generation of peptidomimetic scaf-
folds because as the cyclic proteinogenic amino acid, it has a role in influencing the
biological behavior of peptides due to the secondary structure's inducing and stabi-
lizing properties. In fact, the cyclic structure of proline is capable of restricting the
conformational space of the peptide chain, and many papers have appeared in the lit-
erature focusing on the strategic decoration of the pyrrolidine ring to generate suitable
proline analogs for medicinal chemistry applications [20]. Also, early reports on com-
binatorial approaches described the generation of collections of proline-containing