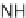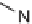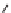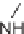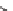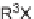Biomedical Engineering Reference
In-Depth Information
SCHEME 6.1
Solid-phase synthesis of a library of 1,4-benzodiazepine-2,5-diones using
-amino acid derivatives as the building blocks.
scaffold capable of being decorated with varying substituents in order to expand the
chemical diversity around it.
Other entries to peptidomimetic combinatorial libraries encompassed known
classes of enzyme inhibitors as powerful tools in rapidly profiling novel protease drug
targets and identifying most effective pharmacophores at their inhibition. Specifically,
the syntheses of phosphonyl acid-based pharmacophore libraries were proposed with
the aim of facilitating the discovery of novel metalloprotease inhibitors of medici-
nal interest; phosph-inyl (-onyl) acids were selected as transition-state analogs and
metal chelators for inclusion in peptidomimetic inhibitors of metalloproteases such
as thermolysin and angiotensin-converting enzyme [12].
Among early examples of small-molecule libraries containing amino acid moi-
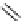
eties, Ellman and co-workers reported a solid-phase synthesis of 1,4-benzodiazepine-
2,5-diones from three commercially available components: namely, anthranilic acids,
-amino esters, and alkylating agents (Scheme 6.1). Moreover, a library of 1,4-
benzodiazepine-2,5-diones was prepared from 11 alkylating agents, 12 anthranilic
acids, and a total of 19
-amino esters coming from nine sets of enantiomeric pairs and
glycine methyl ester hydrochloride, to give 2508 total compounds, or 1320 spatially
separate compounds [13].
Also, another interesting report by Ellman's group is represented by the gener-
ation of a
-turn mimetic library (Scheme 6.2) [14], taking advantage of a facile
intramolecular-cyclative thiol S
N
2 displacement and the simultaneous cleavage of
the molecule from resin to create nine- and ten-membered rings.
In 1996, Gordon et al. reported on a library of heterocycles (4-thiazolidinones,
-
lactams, pyrrolidines, and dihydropyridines) from imines derived from resin-bound
amino acids, following the principles of skeletal diversity from a common starting
substrate [15]. The approach to heterocyclic diversity was conceived by exploiting the
imine chemistry as a useful synthetic strategy and using amino acid building blocks
to give a convenient starting point for exploring the scope of solid-phase organic