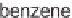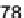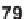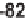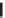Biomedical Engineering Reference
In-Depth Information
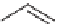
SCHEME 5.30
Synthesis of a mappicine library.
hexamethylditin under sunlamp irradiation conditions. Product mixtures were first
purified by solid-phase extraction (SPE), followed by flash chromatography to afford
the mappicine analogs
82
(19-61%) (Table 5.19). A second-generation library con-
sisting of mappicine ketones was completed through the use of solid-supported oxi-
dants, with resin-bound chromic acid being the most effective.
With the exception of the benzyl-substituted analogs (R
1
benzyl), all map-
picine alcohols
82
cleanly provided the desired ketone products (Scheme 5.31 and
Table 5.20). This radical annulation cascadewas also applied to the camptothecin class
of anticancer agents (Scheme 5.32) [35]. The radical cascade precursors
86
were again
assembled by
N
-propargylation of iodopyridones
84.
In a parallel format, the iodopy-
ridones
84
were exposed to a set of isonitriles (seven analogs), hexamethylditin,
and light.
The streamlined reaction workup and purifications involved automated solid-
phase extractions followed by serial reversed-phase chromatographies to provide
91 homosilatecan analogs
88
in yields ranging from 3 to 64% (Scheme 5.32 and
Table 5.21). A subset of the 91 pentacyclic analogs were deprotected to give the
amino- and hydroxy substituted homosilatecans (Scheme 5.33,
89
and
90
, respec-
tively). Overall, the use of the late-stage cascade radical annulation approach for
generating the polycyclic core of camptothecin-related compounds allowed the syn-
thesis of several analog libraries.
=