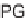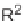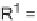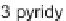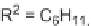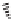Biomedical Engineering Reference
In-Depth Information
SCHEME 5.27
N
-Allylation/Alder-ene and
N
-allylation/formal [2
+
2] domino reactions.
The
N
-allylated intermediates
74
could undergo a homolytic cleavage of the cen-
tral bicyclobutane C-C
-character), affording the putative
biradical intermediates
75
(Scheme 5.28). The resonance-stabilized biradical
75
could
recombine to afford the 3-azatricyclo[5.1.1.0]nonanes
73
. This mechanistic hypothe-
sis was supported by the observation of multiple radical species in ESR-spin trapping
experiments [31].
With this straightforward access to the structurally unique azatricycles, a
-bond (possessing high
5
allyl bromide, Figure 5.2 and Table 5.17) library
was constructed [32]. A larger, tertiary amine containing sublibrary
77
(75 analogs)
×
3 matrix (bicyclobutanes
×
SCHEME 5.28
Proposed mechanism for
N
-allylation/formal [2
+
2] domino reactions.