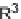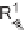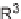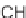Biomedical Engineering Reference
In-Depth Information
SCHEME 5.23
Synthesis of hydroazulenoisoindoles.
Symmetrical dienophiles were utilized for this library to avoid the formation of
mixtures of regioisomers. In all cases, the endo mode of cycloaddition was observed
and confirmed by x-ray crystallographic analysis of select compounds. Additionally,
five hydroazulenoisoindoles
57
were converted into the saturated fused cycloheptanes
58
through diastereoselective hydrogenation.
The Hanson group has published extensively on the preparation of various
substituted sultams (cyclic sulfonamides) [26]. Most recently, a 1,1-dioxido-1,2-
benzothiazoline-3-acetic acid library
63
was prepared using the domino Heck-aza-
Michael (HaM) sequence (Scheme 5.24). The versatility of this protocol is reflected
by sultam formations with three variable points of diversity, originating from the
three starting material components [2-bromobenzylsulfonyl chlorides (
59
) amines
(
60
) and Michael acceptors (
61
)] (Scheme 5.24 and Table 5.15).
The utility of the HaM process was further enhanced by the assembly of a sultam-
containing sublibrary that utilized an additional aryl halogen (
64
), which under
the reaction conditions allowed for a double-Heck alkylation to give analogs
65
(Scheme 5.25 and Table 5.16).
In an effort to provide an alternative, milder route toward benzoxazoles that
allowed for a greater starting material compatibility, Viirre et al. developed a copper-
catalyzed domino annulation using microwave-accelerated conditions [27]. Previous
reports highlighted the intramolecular copper-catalyzed cross-coupling reaction of a
2-haloanilide (isolated
68
) to afford benzoxazole
69
[28], and the authors now applied
two one-pot domino strategies (Scheme 5.26).