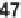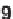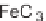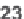Biomedical Engineering Reference
In-Depth Information
SCHEME 5.20
Formation of 2-aminoindole-3-carboxamides
49
.
Deprotonation of cyanoacetamides
46
with NaH and treatment with various 2-
halonitrobenzenes (or pyridines,
47
), followed by an acidic quench, led to crude
reaction mixtures that were subjected to a combination of FeCl
3
(3 equiv) and Zn
dust (10 equiv) at 100
◦
C for 1 h (Scheme 5.20). These Fe/Zn molar ratios were
essential for the formation of the desired 2-aminoindole products
49
.
5.4 TRANSITION-METAL-MEDIATED DOMINO REACTIONS
While investigating selective chemical reactions of trienyl-containing compounds,
Brummond et al. found that by utilizing Davies chemistry [24] to react triene
50
with (
E
)-diethyl-4-diazo-2-pentene dioate and Rh(II) acetate hydroazulenone,
52
was obtained as a single diastereomer (Scheme 5.21) [25]. This formal [4
+
3]
cycloaddition proceeds through the selective cyclopropanation of the vinyl group
to give
cis
-divinylcyclopropane intermediate
51
, which subsequently undergoes a
strain-induced Cope rearrangement to produce racemic cycloheptadiene
52
.