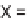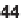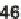Biomedical Engineering Reference
In-Depth Information
SCHEME 5.19
Domino process to oxindoles. (Adapted from [21], with permission; copy-
right
C
2010 American Chemical Society.)
compared to the 2-chloroindole example. This convenient access to polyfunctional
oxindole substrates facilitated the construction of a stereochemically diverse library.
An alternative process to generate 2-aminoindole-3-carboxamides
49
has recently
been reported byWang et al. (Scheme 5.20 and Table 5.13) [22]. Their approach com-
bines a nucleophilic aromatic substitution reaction with a one-pot reductive cycliza-
tion without the isolation of intermediates. The cyanoacetamide starting materials
46
were obtained via amidations of cyanoacetic acid methyl ester (
45
) under neat
conditions [23].
(5.1)