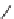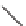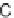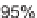Biomedical Engineering Reference
In-Depth Information
SCHEME 5.17
Spirooxindole formation. (Adapted from [21], with permission; copyright
C
2010 American Chemical Society.)
Hydrozirconation of nitriles
42a
and
b
with Schwartz reagent provided metal-
loimines that were subjected to acylation by treatment with hydrocinnamoyl chlo-
ride to provide building blocks
43
and
44.
The configuration of the Friedel-Crafts
cyclization step was controlled by the C2 indole substitution, with the less hindered
chlorosubstituent favoring a pseudoaxial conformation (
A
), whereas the inverted con-
formation (
B
) would be preferred with larger groups at C2 (i.e., OTIPS) (Scheme
5.19). Following hydrolysis of the chloroiminium intermediate (not shown), the
spirooxindoles
43
and
44
were isolated in 83% yield, with a dr
7.3 : 1, from the
2-chloroindole substrate. In the presence of Sc(OTf)
3
, 2-siloxyindole
42b
also gave
high selectivities. In this case, the spirooxindole isomer
44
was the major product
(dr
=
=
5 : 1) formed in a slightly lower ratio of trans : cis amide to benzyloxy groups
42c
SCHEME 5.18
Preparation of cascade precursors.