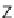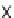Biomedical Engineering Reference
In-Depth Information
SCHEME 5.13
Indanolamide synthesis.
isomers. The syn isomers
39a
are probably formed via a chelation-controlled five-
membered transition state (Scheme 5.15,
TS1
).
The formation of cyclopentyl bicycles via the cascade sequence were slower and
required electron-donating substituents (i.e., methoxyarenes,
36b
or an indole group,
36c
). Unlike the tetrahydronaphthyl-containing examples, these derivatives gave anti
isomers (
39b
and
c
) with high diastereoselectivities (
>
10 : 1, Table 5.12). In addition,
a
-hydroxy amide library composed of
41a
and
b
was prepared by hydrogenolysis
of the benzyl groups (Scheme 5.16).
The nitrile hydrozirconation-acylation-Friedel-Crafts alkylation protocol was
expanded further to produce a stereochemically diverse series of spirooxindoles
43
and
44
(Scheme 5.17) [21]. The pivotal 2-chloro- and 2-triisopropylsiloxyindoles,
42a
and
b
, respectively, were readily prepared from the 3-substituted indole
42c
,
which is available through a Fischer indole synthesis from
N
-benzylphenylhydrazine
and the corresponding aldehyde (Scheme 5.18).
SCHEME 5.14
Preparation of cyanohydrins. (Adapted from [18], with permission; copy-
right
C
2009 American Chemical Society.)