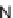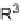Biomedical Engineering Reference
In-Depth Information
TABLE 5.4 Fused Indole Analogs 14 of the Tricyclic
Stemona
Alkaloid Core
Entry
R
R
1
R
2
R
3
Yield (%)
1
H
H
H
H
14a
,76
a
14b
,24
b
2
H
H
i
-Pr
H
14c
,18
b
3
H
H
OMe
H
14d
,37
b
4
H
H
H
Cl
14e
,37
b
5
H
Cl
H
Cl
14f
,26
c
6
Et
H
i
-Pr
H
14g
,77
c
7
Et
H
OMe
H
a
ZnCl
2
/AcOH.
b
TsOH/EtOH.
c
EtOH.
In an effort to develop an efficient method for the synthesis of pyrimidine-
fused heterocycles, Yang et al. envisioned a domino reaction sequence involving the
condensation of
N
-allylaminopyrimidine aldehydes
24
with anilines
25
, followed by
an acid-catalyzed intramolecular inverse electron-demand hetero-Diels-Alder reac-
tion (Scheme 5.10) [15]. As expected, this reaction sequence afforded the desired
cis-configured tetracycles
27
, consistent with the proposed concerted intramolecular
Diels-Alder cycloaddition.
Twenty tetracyclic analogs
27
were prepared using this methodology, with yields
ranging from 49 to 98% (Table 5.7). Whereas this domino sequence demon-
strates a suitable reaction scope, secondary aryl amines required harsher condi-
tions to obtain the desired products (Table 5.7, entries 13 and 14). Several attempts
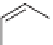
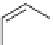
SCHEME 5.7
Quinoline analogs of the tricyclic
Stemona
alkaloid core.