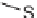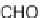Biomedical Engineering Reference
In-Depth Information
SCHEME 5.5
Reductive aminations of tricyclic
Stemona
alkaloid analogs.
This domino reaction sequence was combined into a one-pot four-component pro-
cess by conducting the reactions in toluene in the presence of ammonium chloride
(Scheme 5.9). The addition of ammonium chloride is believed to facilitate imine for-
mation and activation. Toluene was the solvent of choice, as other solvents, such as
THF, MeCN, and benzene, resulted in lower overall yields of the desired pyrrolopy-
ridines
23
. Utilizing this methodology, 12 additional pyrrolopyridines
23
were
synthesized with slightly better overall yields than those of the protocol described
previously (Table 5.6). In general, this reaction sequence is particularly suited for
combinatorial library synthesis due to the level of complexity generated in a min-
imal number of steps combined with the ability to install greater diversity from
commercially available starting materials.
SCHEME 5.6
Carbamate analogs of the tricyclic
Stemona
alkaloid core.