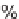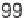Biomedical Engineering Reference
In-Depth Information
SCHEME 5.2
Synthesis of an isoquinolone library.
(Scheme 5.6). A 6
isocyanate) matrix produced 22 new analogs
(2 to 98% yields, Table 5.3). Several of these scaffolds were manipulated further
to incorporate fused indole or quinoline functionalities that are present in many
biologically active substrates (Table 5.4) [12]. For example, Fischer indolization
procedures were successfully applied to the scaffold
11
to give the pentacycles
14
,
whose configurations were elucidated by x-ray crystallography.
The fused quinolines
18
were prepared in good yields (48 to 86%) using a modified
Friedlander synthesis (Scheme 5.7) [13]. In these cases, the ketones were treated with
the substituted 2-aminobenzaldehydes, generated in situ, in the presence of ZnCl
2
and molecular sieves to provide five fused heterocycles
18
.
Janvier et al. followed a guiding principle combining the use of multicomponent
reactions and domino processes to develop a highly efficient synthesis of a com-
binatorial library [14]. They coupled a three-component reaction successfully with
an acylation/IMDA/retro-Michael cycloreversion triple domino sequence to afford
pyrrolo[3,4-
b
]pyridin-5-ones
23
(Scheme 5.8) [14].
Initial studies toward the formation of library
23
via a three-component con-
densation of an aldehyde, an amine, and the
×
4 (alcohol
×
-isocyanoacetamide
19
in methanol
were focused on 5-aminooxazole
20
. The use of
-isocyanoacetamides
19
in place
of commonly used
-isocyanoacetate in this Ugi type of transformation facilitated
the desired cyclocondensations in good overall yields (60 to 96%). Acylation of the
secondary amine to amide
21
followed by an intramolecular Diels-Alder cycload-
dition produced the bridged tricycles
22
. Finally, a base-mediated retro-Michael
cycloreversion afforded the desired pyrrolopyridines
23
(Table 5.5).