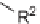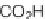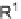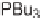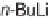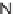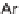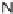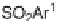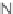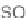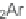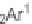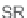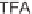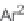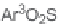Biomedical Engineering Reference
In-Depth Information
SCHEME 4.29
Library synthesis through combinatorial scaffolding on a solid support.
of phosphine-catalyzed annulation reactions, Tebbe, and Diels-Alder reactions and,
in some cases, hydrolysis. We unequivocally established the relative configuration of
each scaffold, with the exception of compound
99a
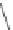
, through x-ray crystallographic
analysis.
Using solid-phase synthesis for the generation of a library with high skeletal
diversity is another key feature of our phosphine catalysis-based DOS strategy
(Scheme 4.29) [63,64]. Split-pool synthesis on a solid support is one of the fastest
and most efficient approaches to generating a large number of spatially segregated
compounds. We coupled the hydroxyl groups of SynPhase lanterns of Wang resin
106
to the allenoic acids
105
in the presence of Mukaiyama's reagent. The subse-
quent phosphine-catalyzed [3
2] annulations of the resin-bound allenoates
107
with the
N
-sulfonylimines
108
gave the resin-bound dihydropyrroles
110
and
tetrahydropyridines
114
, which we cleaved from the resin using 2.5% TFA in CH
2
Cl
2
to provide the carboxylic acids
111
and
115
, respectively, in good to excellent yields
with high diastereoselectivities after chromatographic purification.
We employed the common chemical functional group (
+
2]/[4
+
-unsaturated ester) of
the heterocycles
110
and
114
in the next scaffold-generating split step. The Michael
,