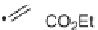Biomedical Engineering Reference
In-Depth Information
SCHEME 4.21
Synthesis of highly functionalized tetrahydropyridines.
nucleophilic aromatic substitution and desulfonylation affords
64
; intramolecular
conjugate addition of the amide
64
and
-elimination of the phosphine yields the
final product 5
9a
.
Although the reaction is catalytic in nature, 1 equiv of triphenylphosphine can
be used to expedite the transformation. Under the optimized reaction conditions,
we tested a range of aziridine derivatives as partners for the [3
3] annulations.
Phenyl aziridines featuring both electron-rich and electron-deficient substituents at
the ortho, meta, and para positions are amenable to the reaction, giving products
in good to excellent yields. With aryl-substituted aziridines, the reaction produced
tetrahydropyridines favoring 1,2-trans, whereas alkyl-substituted aziridines produced
1,3-cis isomers preferably (Scheme 4.21 and Table 4.9).
+
4.2.6 Phosphine Organocatalysis of Allenes with Dinucleophiles
Whereas phosphines catalyzes Michael additions of nucleophiles onto activated
alkenes [86], nucleophiles undergo
-umpolung addition onto activated allenes
-umpolung additions onto
[87-89] and
-alkyl allenoates [90,91] in the presence of
phosphine catalysts. Considering that the
-umpolung addition product of a nucle-
ophile onto an allenoate is a
,
-enoate, we envisioned the possibility of tandem
-umpolung-/Michael addition of a dinucleophile onto an allenoate [92]. Indeed, we
isolated the benzomorpholine
66
in 88% yield when using
N
-tosyl-2-aminophenol
65a
as the dinucleophile reaction partner in the presence of triphenylphosphine
(Scheme 4.22). Switching the catalyst
to trimethylphosphine, however,
led to
TABLE 4.9 Highly Functionalized Tetrahydropyridines
R
1
R
2
Entry
Yield (%)
trans : cis
1
H
2-Me-Ph
88
90 : 10
2
H
3-Me-Ph
82
86 : 14
3
H
4-Me-Ph
64
89 : 11
4
H
2,5-Me
2
-Ph
98
92 : 8
5
H
4-F-Ph
76
88 : 12
6
H
3-Cl-Ph
86
83 : 17
7
Me
H
66
41 : 59
8
H
H
37