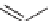Biomedical Engineering Reference
In-Depth Information
TABLE 4.8 Functionalized Dihydro-2-pyrones
Entry
R
2
Yield (%)
1
3-CN-Ph
83
2
4-CF
3
-Ph
63
3
3-CF
3
-Ph
74
4
2-CF
3
-Ph
58
5
3-Me-Ph
36
available starting materials was recognized immediately: Pollastri and his colleagues
employed our method to prepare dihydro-2-pyrone suppressors of TNF
[84].
4.2.5 Phosphine Organocatalysis of Allenes with Aziridines
Aziridines are extremely versatile synthetic building blocks in modern synthetic
chemistry [85]. By incorporating them in the phosphine organocatalysis of allenes,
we developed a novel [3
3] annulation to generate highly functionalized tetrahy-
dropyridines [55]. In the mechanism proposed (Scheme 4.20), addition of triph-
enylphosphine to the allenoate forms the corresponding phosphonium zwitterion
60
, which undergoes proton transfer to provide the vinylogous ylide
61
. The addi-
tion of the intermediate
61
to the phenyl aziridine occurs to form
62
, which is
converted to the phosphonium dienolate
63
through proton transfer. Intramolecular
+
CO
2
Et
NO
2
O
2
N
Ph
HN
1 eq. PPh
3
+
N
CO
2
Et
Ph
S
CH
2
Cl
2
, rt
CO
2
Et
CO
2
Et
O
O
57
59a
58a
PPh
3
PPh
3
CO
2
Et
EtO
2
C
CO
2
Et
CO
2
Et
Ph
PPh
3
NO
2
+
PPh
3
60
HN
O
+
CO
2
Et
S
PPh
3
58a
64
O
NO
2
N
EtO
2
C
Me
Me
CO
2
Et
O
O
+
Ph
3
P
Ph
S
PPh
3
EtO
2
C
H
61
Ph
EtO
2
C
CO
2
Et
NO
2
63
62
SCHEME 4.20
Phosphine-catalyzed [3
+
3] annulations with aziridines.