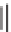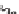Biomedical Engineering Reference
In-Depth Information
s
-
trans
-isomer
R
1
O
2
C
R
2
OR
1
PR
3
CO
2
R
1
+
+
O
O
-
O
-
PR
3
PR
3
R
2
R
2
CO
2
R
1
O
49
48
50
51
R
2
CHO
R
1
OH + PR
3
R
2
O
O
49
PR
3
R
2
O
-
R
2
OR
1
OO
47
CO
2
R
1
55
+
R
3
P
O
R
2
-
O
O
+
+
53
52
54
PR
3
R
1
O
-
PR
3
s
-
cis
-isomer
56
OR
1
SCHEME 4.16
Phosphine organocatalysis of allenes with aldehydes. (Adapted from [53],
with permission; copyright
C
2008 American Chemical Society.)
i
-PrO
2
C
O
cat.PMe
3
CHCl
3
, rt
O
CO
2
i
-Pr
+
Ar
H
Ar
O
Ar
47a
49a
51a
SCHEME 4.17
Synthesis of 1,3-dioxan-4-ylidenes.
groups afforded the desired products in good to excellent yields with good to excellent
stereoselectivities favoring the
E
-isomers. Less reactive electron-rich benzaldehydes
afforded moderate reaction yields (Scheme 4.17 and Table 4.6).
When using relatively bulky tricyclopentylphosphine as the catalyst, the
phosphine-catalyzed reactions of allenoates and aldehydes provided 2-pyrones
as products. Ethyl 2,3-butadienoate provided yields greater than those of other
2,3-butadienoates. Various aromatic aldehydes bearing electron-withdrawing and
TABLE 4.6
1,3-Dioxan-4-ylidenes
Entry
Ar
Yield (%)
E:Z
1
4-Py
99
8 : 1
2
3-Py
96
9 : 1
3
4-CF
3
-Ph
99
7 : 1
4
3-NO
2
-Ph
97
7 : 1
5
2-Cl-Ph
64
8 : 1
6
Ph
54
only
E
7
3-OMe-Ph
47
only
E