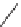Biomedical Engineering Reference
In-Depth Information
O
R
R
H
N
N
cat. PPh
3
benzene, 60
°
C
CO
2
Et
O
O
CO
2
Et
+
O
H
99%, R = Ph
95%, R = Et
4a
35
36
SCHEME 4.11
Allene-maleimide [3
+
2] annulations.
This highly efficient reaction has also worked well for resin-bound allenoates, allow-
ing us to use it to generate a library of 145 bicyclic succinimides.
4.2.3.2
2] Annulation to Form Coumarins
Another way to
avoid regioscrambling, and thereby obtain a single product from the allene-alkene
[3
Intramolecular [3
+
2] annulation, is to perform the reaction in an intramolecular setting. The geo-
metric constraint inherent in the starting material can control the reaction mode so
that only one pathway will dominate. We synthesized compounds
37
from salicy-
laldehydes through Wittig reactions followed by coupling with 3-butynoic acid under
the influence of Mukaiyama's reagent. Treatment of
37
with 20 mol% of tributylphos-
phine in THF at room temperature for 6 h produced the tricyclic dihydrocoumarins
38
in good to excellent yields as a single diastereoisomers. Salicylaldehydes featuring
either electron-donating or electron-withdrawing substituents were readily converted
into the coumarin derivatives
38
(Scheme 4.12) [49].
From an extensive investigation of the role played by the activating substituents
on the olefin moiety, we found that the reaction of the nitrostyrenyl derivative
39
with
triphenylphosphine in benzene provided the nitronate
40
in 58% yield. The nitronate
40
underwent 1,3-dipolar cycloadditions with a variety of commercially available
alkenes, providing highly functionalized tetracyclic coumarins
41
in excellent yields
(83 to 97%) with high exo selectivity (exo/endo
+
=
9-11 : 1) and exclusive facial
selectivity (Scheme 4.13).
4.2.3.3
2] Annulation with Arylidenemalononitriles to Form Cyclohexenes
In the imine [4
[4
+
+
-alkyl allenoates reacted with
N
-tosylimine to form
tetrahydropyridines. The reaction works well for a variety of
N
-sulfonylimine elec-
trophiles [47]. To expand the utility of the [4
2] annulation,
+
2] annulation, we became interested in
CO
2
Et
EtO
2
C
cat. PBu
3
THF, rt
R = 3-Me, 98%;
R = 3-OMe, 74%;
H
R = H, 96%;
R
R
O
O
R = 4-OMe, 94%;
R = 5-Br, 93%;
O
O
R = 5-OMe, 70%.
R = 5-F, 91%;
37
38
SCHEME 4.12
Intramolecular [3
+
2] annulations to form highly functionalized coumarins.