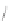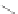Biomedical Engineering Reference
In-Depth Information
O
Me
O
cat. PBu
3
CH C
2 ,
rt
N
•
CO Et
2
NN
+
N
-
+
CO Et
2
Ar
7a
15a
16a
Ar=
p
-NO C H
Ar
26 4
PBu
3
PBu
3
O
O
+
PBu
3
15a
N
N
CO Et
-
-
2
N
N
CO Et
2
PBu
3
PBu
3
CO Et
2
+
Ar
Ar
11
23
24
SCHEME 4.6
Phosphine-catalyzed [3
+
2] annulations with azomethine imine.
O
R
2
R
2
O
Phosphin
e
CH C
2 ,
rt
N
N
N
+
+
N
R
1
•
CO Et
2
CO Et
2
15
R
1
7
16
SCHEME 4.7
Synthesis of highly functionalized tetrahydropyrazolopyrazolones.
TABLE 4.3 Highly Functionalized Tetrahydropyrazolopyrazolones
Phosphine
(20 mol%)
Time
(h)
Yield
(%)
R
1
R
2
Entry
1
Ph
H
PBu
3
24
97
2
4-OMe-Ph
H
PBu
3
30
85
3
4-CF
3
-Ph
H
PBu
3
24
98
4
3-NO
2
-Ph
H
PBu
3
24
97
5
2-Me-Ph
H
PBu
3
48
70
6
2-Naphthyl
H
PBu
3
24
96
7
4-Py
H
PBu
3
20
86
8
2-Furanyl
H
PBu
3
20
99
9
4-NO
2
-Ph
Me
PMe
3
20
96
a
10
4-NO
2
-Ph
t
-Bu
PMe
3
24
98
a
11
4-NO
2
-Ph
2-Me-Ph
PMe
3
20
95
12
4-NO
2
-Ph
4-F-Ph
PMe
3
15
99
a
At 40
◦
C.