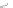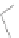Biomedical Engineering Reference
In-Depth Information
R
CO
2
Me
cat. PPh
3
benzene, rt
•
Ts
CO
2
Me
+
R
N
N
Ts
1
2
3
SCHEME 4.1
Xu and Lu's [3
+
2] annulation with
N
-tosylimines.
Ts
N
R
Ar
cat. PBu
3
benzene,rt
•
CO
2
Et
Ts
R
+
Ar
N
CO
2
Et
4
5
6
SCHEME 4.2
Synthesis of tetra-substituted dihydropyrroles.
4.2 DOS USING PHOSPHINE ORGANOCATALYSIS
4.2.1 Phosphine Organocatalysis of Allenes with Imines
4.2.1.1
2] Annulation with N-Tosylimines to Form Dihydropyrroles
In
1997, Xu and Lu established the construction of dihydropyrroles
3
as a single annula-
tion product when using
N
-tosylimines
2
as reaction partners in triphenylphosphine-
catalyzed [3
[3
+
2] annulations (Scheme 4.1) [70]. As part of a program to synthesize a
library of heterocycles, we decided to employ
+
-substituted allenoates
4
to maximize
the diversity in the dihydropyrrole products. The mechanism of the [3
+
2] annulation
dictates that the
-substituted allenoates
4
should provide tetra-substituted dihydropy-
rrole products
6
and, therefore, increase the versatility of the reaction (Scheme 4.2
and Table 4.1) [46].
When we subjected
-ethyl allenoate to Xu and Lu's original reaction conditions
with
N
-tosylbenzaldimine, we obtained only 44% of the desired 2,5-disubstituted
dihydropyrrole as a 15 : 1 mixture of diastereoisomers. We hypothesized that the
sterically more-hindered
-substituted allenoate would require a more nucleophilic
TABLE 4.1 Tetra-Substituted Dihydropyrroles
Entry
R
Ar
Yield (%)
cis : trans
1
Me
Ph
89
91 : 9
2
Et
Ph
99
95 : 5
3
n-
Pr
Ph
98
96 : 4
4
i
-Pr
2-F-Ph
97
cis only
5
i
-Pr
4-CF
3
-Ph
96
cis only
6
Ph
3-Cl-Ph
99
cis only
7
Ph
4-OMe-Ph
99
cis only
8
t
-Bu
3,4-Cl
2
-Ph
>
99
cis only
9
t
-Bu
1-Naphthyl
>
99
cis only