Biomedical Engineering Reference
In-Depth Information
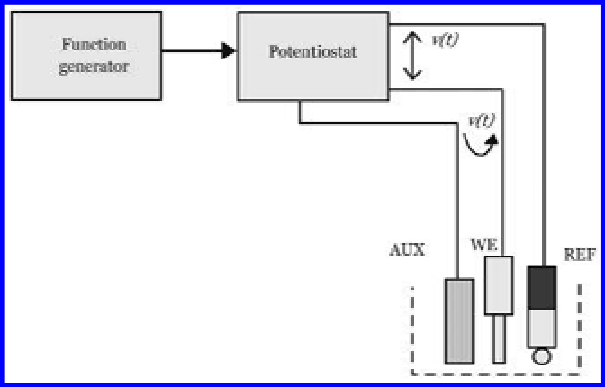
A basic component included in a general electrochemical measurement system
is the electricity generating device, i.e., potentiostat, which in voltammetry injects
current into an auxiliary electrode (AUX). This means that the measurement closes
the current loop via a working electrode (WE) in order to impose a known differ-
ence of potential between the working and reference electrodes (REF), as shown in
Fig. 6.18.
Further, in voltammetry an applied simple or advanced waveshaped voltage,
v(t), is imposed across the working electrode and the reference electrode, while
measuring the resulting response. The applied voltage will close the measuring
loop and give rise to the loop current, i(t), which is measured.
In Fig. 6.18, a simplified schematic diagram of the experimental design is pro-
posed. The simplified but illustrative design is normally in the extensive exper-
iments made by a measurement cell comprising one working electrode, made of
a metal that in normal devices are Au, Re, Pt, Pd. A corresponding experimental
sensor is illustrated in Fig. 6.19.
However, in a simplified design for exploring the principal operation, the use
of an electrode made of copper will satisfy the fundamental needs to experimen-
tally demonstrate the function of a water safety system. As the reference electrode,
or in this case, a spoon of stainless steel (e.g., 18-12) is sufficient to experience the
system performance.
A simple experiment that can be easily performed is shown in Fig. 6.20. The
electrodes are placed in a plastic-beaker of a tea cup size. The electrodes, in this
experiment consist of a spoon and a fixed piece of copper wire. Due to chemi-
cal properties which will affect the measurement, we aim to let the same area of
the electrode during the entire experiment be in contact with the liquid. Also to
achieve the best possible response there may be a need to adjust the electrodes
mutual distance to each other. By applying a voltage pulse sequence, for exam-
Figure 6.18.
The electrochemical measurement principle shown in a simple experimental
system.







Search WWH ::

Custom Search