Biomedical Engineering Reference
In-Depth Information
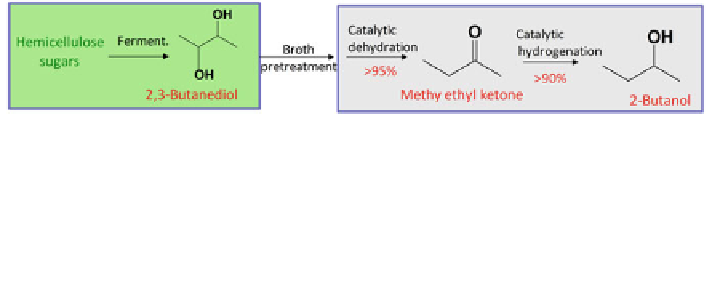
Fig. 4 An integrated 2,3-butanediol (2,3-BD)-methyl ethyl ketone (MEK)-2-butanol coupled
production route using hemicellulose sugars as substrates. The process can be divided into three
steps: (1) fermentation to convert hemicellulose sugars to 2,3-BD (*50% yield, 40%);
(2) Chemical dehydration of 2,3-BD to form MEK ([95% yield), using dehydration catalysts
sulfuric acid, acidic oxides, zeolites, etc.; (3) hydrogenation of MEK to produce 2-butanol ([90%
yield), using hydrogenation catalysts mixed oxides, Raney Ni, Pd/C, Ru/oxides, supported Cu,
supported Fe, etc.
promoted renewed studies of ABE fermentation. A higher concentration of 20 g/L
has reportedly been achieved [
98
]. Iso-butanol exists in very small quantities in the
''fusel oil'' of beverage alcohol fermentation. Genetically modified microbial cells
have achieved higher yields. It is not as toxic as n-butanol but the final concentration
is still reported to be no more than about 60 g/L [
99
,
100
]. t-Butanol is a product of
petrochemical processing. No known biological process can produce t-butanol at
present. 2-Butanol could be made from fermentation using hemicellulose-derived
pentoses (C5) and also hexoses (C6) to produce the intermediate 2,3-BD that has an
inhibitory effect only after the concentration exceeds 11% [
65
]. The process for
bio-based 2-butanol production can be divided into three steps: (1) fermentation to
convert sugars to 2,3-BD (*50% yield, 40%); (2) chemical dehydration of 2,3-BD to
form MEK, a marketable intermediate product ([95% yield); (3) hydrogenation of
MEK to produce 2-butanol (theoretically, 100 g of MEK can produce 103 g of
2-butanol; the practical yield is 90% of the theoretical). Therefore, 100 g of C5 or C6
sugars from hemicelluloses hydrolysis will yield 35.2 g of 2-butanol, or approxi-
mately 1 ton of 2-butanol from 3 tons of sugars from starch, cellulose and
hemicellulose, or 100 gallons of 2-butanol per ton of sugars. That is to say, the bio-
based 2-butanol route is more competitive than the traditionally used n-butanol or
iso-butanol fermentation process, as the latter two butanol isomer fermentations not
only have a lower final product concentrations but also a relatively lower yields from
the sugars. The coupled bio-based 2-butanol production route from lignocelluloses
using fermentatively-produced 2,3-BD as intermediate is therefore economical to
some extent (Fig.
4
). In addition, the fermentatively produced 2,3-BD could be
directly catalyzed to 2-butanol using a heterogeneous catalyst system that can
function both as an acid catalyst and as a hydrogenation catalyst [
101
]. With
emerging synthetic biology tools, the 2,3-BD biosynthetic pathway and the 2,3-BD
to 2-butanol pathway existing in some lactic acid bacteria could be constructed into a
new minimal genome chassis to form a homo-fermentative 2-butanol-producing
strain. The de novo biosynthesis of 2-butanol would then be achieved [
102
-
105
].
Search WWH ::

Custom Search