Biomedical Engineering Reference
In-Depth Information
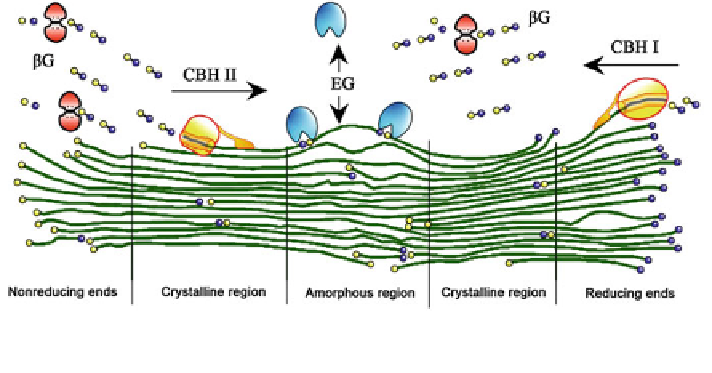
Fig. 2 Mechanism of cellulose biodegradation. CBH cellobiohydrolase (or exo-b-glucanase),
EG endo-b-glucanase, bG b-glucosidase
the behavior of the complicated enzymatic lignocellulose degradation is difficult to
model and prone to experimental errors.
CBHs have a higher reactivity on crystalline cellulose than EGs. Both CBHs and
EGs digest long polysaccharide chains into smaller fragments, thereby reducing the
DP of the substrate. CBHs have been shown to be primarily responsible for solubi-
lization of cellulose and only decrease the DP incrementally, whereas EGs decrease
DP significantly but only play a minor role in cellulose solubilization [
27
]. Research
on the cellulolytic enzyme system in T. reesei suggested that CBHs are the most
abundant proteins in this system, representing more than 37% of the total protein
content [
17
]. Therefore, understanding the structures of CBHs, as well as their cat-
alytic mechanisms, is important when attempting to improve the enzymatic hydro-
lysis rate of lignocellulose [
24
]. Using CBH I, which catalyzes the cleavage of
cellulose from the reducing end of the polysaccharide chain as an example, we
attempt to address the complexity of the mechanism of these enzymes and also the
synergistic relationship between different domains in these enzyme molecules.
Previous studies on CBH I identified two functional domains in this enzyme:
the cellulose-binding domain (CBD; also referred to as the cellulose-binding
module) and the catalytic domain (CD), which are linked by a flexible linker [
18
].
Further structural investigations revealed a synergistic relationship between these
two domains, whose collaborative efforts lead to crystalline cellulose degradation
[
28
,
29
]. On the basis of these observations, we suggested an intramolecular
synergic model for CBH I during its degradation of cellulose, which is shown in
Fig.
3
in detail.
In this synergic model, multiple enzymes are involved in the degradation of
cellulose, of which EGs first randomly cleave the internal amorphous regions,
thereby generating reducing ends in the polysaccharide chains in preparation for
CBH I degradation [
18
,
27
]. CBH I is subsequently recruited to these sites by the
binding of the CBD to the hydrophobic surfaces of crystalline cellulose [
30
].
It was suggested that the CD assists in this recruitment process and synergistically
Search WWH ::

Custom Search